Navigate BioPharma Services CLL-MRD Method Summary
Intended Use
The Chronic Lymphocytic Leukemia Minimal Residual Disease by Multiparametric Flow Cytometry (B‑CLL MRD MFC) Test is a quasi-quantitative lab-developed in vitro test that utilizes the core set of cell differentiation markers utilized as part of an internationally harmonized approach for MRD monitoring in bone marrow and peripheral blood of B-CLL patients (Rawstron, 2007, 2016). Each test result is reviewed by a board-certified hematopathologist. The B‑CLL MRD MFC test results are utilized in conjunction with standard clinical response criteria to interpret treatment efficacy.
Method Background and Design Principles
While the eradication of minimal residual disease (MRD) in chronic lymphocytic leukemia (CLL) predicts for improved outcome, a wide variety of MRD techniques makes it difficult to interpret and compare different clinical trials. In an effort to develop a standardized flow cytometric CLL-MRD assay, an internationally recognized group of laboratories assessed 50 CLL-specific antibody combinations and identified three antibody combinations (CD5/CD19 with CD20/CD38, CD81/CD22 and CD79b/CD43) that showed lowest inter-laboratory variability and false positive rate for the identification of B-CLL cells (Rawstron, 2003). Optimal sensitivity and specificity were observed when at least 50 B-CLL events were present in gates created by at least two of the three antibody combinations (namely, CD22/CD81/CD19/CD5 and CD43/CD79b/CD5), and a minimum of 500,000 events were acquired to reach a sensitivity of 0.01%. Experienced operators demonstrated an accuracy of 95.7% (specificity 98.8%, sensitivity 91.1%) in 141 samples with 0.01–0.1% CLL. There was close correlation and 95% concordance with RQ-ASO IgH-PCR for detection of CLL above 0.01%. This international laboratory group also concluded that this flow cytometry approach is applicable to all sample types and therapeutic regimes, and sufficiently rapid and sensitive to guide therapy based on survival outcomes observed in multiple clinical trials (Moreton, 2005, Bottcher, 2012, Kwok, 2016).
More recently, the CLL international flow cytometry group developed a single eight-color combination comprising CD19, CD20, CD5, CD43, CD79b, CD81, CD22 and CD3 based on the merging of the markers utilized in the previously harmonized approaches (Rawstron, 2016). The goal of introducing this single tube design was to develop a standardized method for MRD analysis that utilizes lower bone marrow specimen and reduce interpretation errors associated with combining data from multiple tubes. Additionally, CD3 antibody was introduced to help exclude contaminating T-cells from B-cells. This single tube method showed good concordance both with previously harmonized MRD MFC approaches as well as high-throughput sequencing of IgH-CDR3 at the 0.010% (10− 4) level (Rawstron, 2016), the MRD threshold defined in the 2008 International Workshop on CLL guideline. Based on these findings, we designed a 2-tube, 8-parameter assay to reliably monitor MRD in global clinical trials enrolling CLL patients. The first tube incorporates antibodies directed against the core set of eight cell differentiation markers identified by international flow cytometry group for identification of the broadest group (>95%) of B-CLL cases, while the second tube contains viability dye dedicated to confirming specimen integrity in a global clinical trial setting (see Table 2).
Table 2: B-CLL MRD MFC Assay Design
| Marker 1 | Marker 2 | Marker 3 | Marker 4 | Marker 5 | Marker 6 | Marker 7 | Marker 8 | |
| Tube 1 (MRD core panel) | CD5 | CD3 | CD81 | CD79b | CD22 | CD19 | CD43 | CD20 |
| Tube 2 (Viability) | 7-AAD |
Navigate BioPharma Specimen Handling
Bone marrow aspirates and peripheral blood are collected in sodium-heparin tubes and shipped to the Navigate clinical testing laboratory at ambient (18-22 OC) temperature. All specimens received are visually inspected and description of sample integrity, including clotting and/or hemolysis are documented. Ambient specimen stability is 5 days, and the majority of the specimens collected globally were tested within 72 hours of collection. Specimens received outside the stability window were evaluated by a board-certified pathologist and results reported with a “disclaimer”.
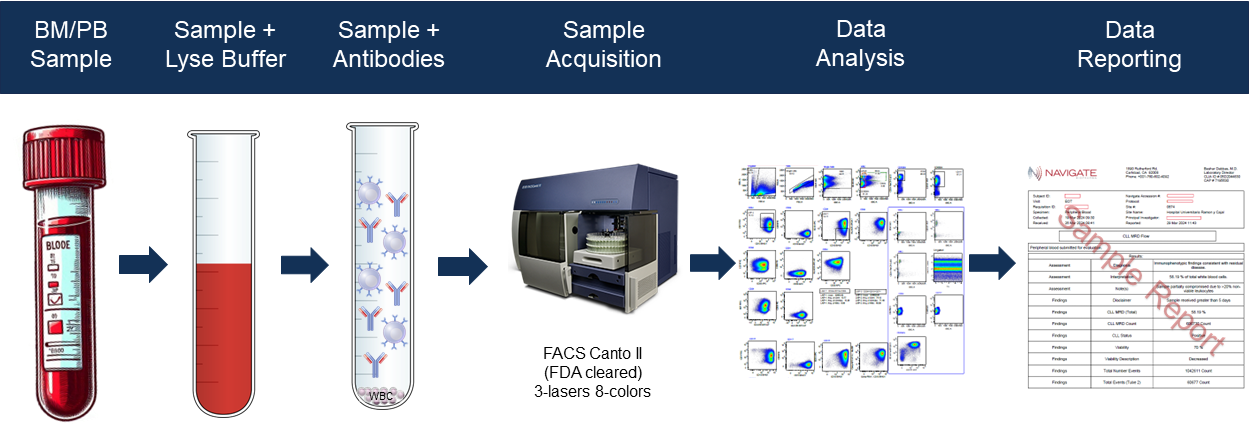
Figure 1. B-CLL MRD MFC Assay Workflow
Interpretation Principles
CLL cells identified based on differential expression of a core set of markers described in Table 3 using a gating methodology developed by expert scientists and board-certified hematopathologist. A specimen is considered MRD-positive if there is at least 0.01% B-CLL of total WBC, and at least 20 clustered events, per industry guidelines (Rawstron, 2016).
Table 3: Expression of Markers used to Differentiate CLL from Normal Cells in MRD Analysis
Antigen | Typical expression in CLL 1 | Control populations in normal peripheral blood | |
|---|---|---|---|
Positive | Negative | ||
CD5 | Positive (>20%) | CD3+ T cells | CD19+ B cells |
CD20 | Weak | CD19+ B cells | CD3+ T-cells |
CD43 | Positive (>20%) | CD3+ T cells | CD20+ B cells |
CD79b | Weak | CD20+ B cells | CD3+ T-cells |
CD81 | Weak | CD3+ T cells | Granulocytes |
1For the typical expression pattern, 'positive' indicates percentage of cells positive compared with control, and 'weak' indicates at least 20% reduction in fluorescence intensity relative to the median expression observed with a reference population of polyclonal B cells using the same antibody. Flow cytometry instruments are calibrated to provide separation of CLL cells from normal B cells in >95% of cases (see Figure 1 above) with a preferred relative fluorescence intensity being the level at which >99% of cases have optimal separation of CLL cells from normal B cells (adapted from Rawstron, 2016).
Analytical Performance Summary
The assay validation focused on evaluation of specificity, sensitivity (LLOQ, LOD, & LOB), precision, and accuracy (concordance with reference laboratory) as key criteria for assessing assay performance. A combination of freshly collected blood and bone marrow specimens from CLL patients and healthy donors were utilized to demonstrate analytical performance. Analytical validation plan and specimen numbers were designed based on recommendations by clinical cytometry consortia (Wood, 2013) and CLSI H62 (Wayne, 2020). The results of the assay validation activities are summarized in Table 5 below and complete details are described in an independent assay validation report.
Table 5: B-CLL MRD MFC Assay Validation Summary
| Validation Parameter | Experimental Design* | Acceptance Criteria | Results |
|---|---|---|---|
| Analytical Specificity | Antibody specificity verified on known negative and positive control cells at the pre-determined optimal concentration.
Assay specificity verified on 8 PB and 3 BM specimens from normal subjects (diagnosed as “negative” for CLL). | Optimal antibody amount on the positive control must be negative on the negative control.
Normal subjects must be MRD negative (<0.01% CLL disease burden). | All antibodies tested negative, at the optimal concentration, on the negative control cell types.
All 11 subjects were MRD negative with residual disease burden below <0.01%. |
| Functional Sensitivity (LLOQ/MRD Cut Point) | Twelve confirmed B-CLL patient specimens were spiked into normal donor PB to achieve eight 2-fold dilutions (including blank) ranging from 10% to 0.0001% of total WBC. The specimens were created using admix approach described in Wood 2013. | 50 Minimum clustered events within target B-CLL gate and ≤35% CV achieved amongst replicates.
| LLOQ = 0.01%. |
| Analytical Sensitivity (LOB/LLOD) | Specimens from functional sensitivity study described above were used to estimate LOB and LLOD. | Observational Study. | LOB = 0.002% (mean + 1.645 SD of 36 blank measurements).
LLOD = 0.003% (mean of 36 blank measurements + 3 x SD). |
| Analytical Precision | Three native B-CLL clinical specimens with varying tumor frequencies were used to assess precision, tested in triplicate across intra-assay, inter-assay, inter-operator and inter-instrument studies. | ≤25% CV (≤35% CV at LLOQ). | Mean Imprecision (%CV range = 0.00 to 10.88) across three tumor levels and 63 measurements. |
| Accuracy (concordance with reference laboratory) | 5 B-CLL patient specimens were spiked into normal donor (without presence of B-CLL) PB and BM to achieve tumor levels ranging from 10% to 0.01% and 5 normal donor specimens were evaluated between Navigate BP and reference laboratory by the same 8-color flow cytometry assay. | Qualitative Concordance for MRD interpretation ≥ 90%. Quantitative Equivalence within ± 30% of tumor levels. | QualitativeConcordance for MRD interpretation = 96% (26 out of 27 specimens). Quantitative Equivalence = 26 out of 27 samples showed equivalent tumor levels within ± 30% across both laboratories (correlation coefficient Pearson R > 0.9). |
| Specimen Stability | Native peripheral blood specimens from a minimum of ten B-CLL patients containing varying levels of leukemic cells are stored at ambient temperature and tested daily up to seven days. | The latest time point where at least 80% of samples underwent ≤20% change from Day 1 (baseline). | 20 of 25 (80%) passed CLSI H62 recommended criteria at Day 5. |
| Linearity of Population Frequency | Specimens from functional sensitivity study described above were used to calculate linearity using observed vs. expected tumor cell plot. | Observational Study. | Linear regression was observed to be (R2)= 0.9728 indicating good correlation (n = 210 measurements). |
| Cell Carryover | Two specimens, one containing high frequency B-CLL tumor burden and a second lacking tumor cells (PBS) were tested in triplicates to verify carryover events between sample acquisitions. | Carryover should not exceed 20% of LLOQ or 10 events in the LAIP gate. | No carryover that exceeds 20% of LLOQ. |
| Impact of WBC Counts on MRD Interpretation | Three native B-CLL patient specimens were spiked into healthy peripheral blood at a target concentration of 0.01% (LLOQ). Total WBC events ranging from 0.025 to 1.0 million were acquired. | Spiked B-CLL frequency (0.01%) should remain within ≤35% CV of the highest number of WBC acquired (1 million) across the range of WBC events. | Based on the results, minimum WBC events required for reliable MRD interpretation was found to be 200,000. |
| Impact of # of WBCs used for Antibody Staining on MRD Interpretation | B-CLL specimens with total WBC event counts of 3 million, 2 million, 1 million, 0.5 million and 0.25 million were stained with antibody cocktails to evaluate impact on MRD interpretation. | Frequencies of B-CLL cells should remain within ≤25% CV of the B-CLL frequencies observed in the 3 million tube, which was used as the operating standard for the original test method. | Frequency of B-CLL in each specimen showed ≤25% CV of the 3 million tube indicating no impact of antibody staining on WBCs used in the range of 3 to 0.25 million. |
| Assessment of Investigational Agent Interference on MRD Interpretation | Twenty (20) randomly selected paired patient specimens at screening (pre-treatment) and Cycle 19, Day 1 (post treatment) from clinical study were analyzed for change in median fluorescent intensity (MFI) on critical markers used to define CLL MRD. | Observed change in fluorescence of critical markers in post treatment specimens must not interfere with ability to reliably identify (gate) abnormal B-cells (CLL MRD). | While the investigational agents in clinical study had variable effect on MFI of critical markers used to define CLL MRD, even the highest change in MFI were not significant enough to alter accurate identification of CLL MRD. |
Discussion & Clinical Experience
The validation data summarized in this document provide evidence that the MFC Method to Assess MRD in bone marrow and peripheral blood from CLL patients met analytical performance standards recommended by clinical cytometry consortia and CLSI guidance documents. Furthermore, utilization of a core set of markers previously proven to correlate with survival outcomes indicate that the MRD results generated by Navigate BioPharma are suitable for interpretation of treatment efficacy. Over the past decade this validated method has been utilized for understanding depth of tumor clearance and length of remission in multiple Phase 2 and Phase 3 clinical trials of BTK inhibitors and other small molecular inhibitors outlined in Table 6 below. We believe incorporating MRD flow biomarker assays in clinical trial programs for blood cancers can provide valuable insights into treatment response, patient risk stratification, and personalized therapeutic approaches, ultimately leading to improved patient outcomes.
Table 6: Summary of Clinical Trial Experience with B-CLL MRD MFC Assay
| Parameter | Trial #1 | Trial #2 | Trial #3 | Trial #4 |
| Disease | CLL/SLL | CLL/SLL | CLL | CLL |
| Study Population | 200 | 320 | 626 | 515 |
| Phase | III | II | III | III |
| Investigational Agents | ibrutinib | ibrutinib | lenalidomide | lenalidomide |
| Specimen Type | PB and BM | PB and BM | PB and BM | PB and BM |
| # of Cases Reported | 2144 | 3202 | 957 | 644 |
References
- Bottcher, S., Ritgen, M., Fischer, K., Stilgenbauer, S., Busch, R.M., Fingerle-Rowson, G., Fink, A.M., Buhler, A., Zenz, T., Wenger, M.K., et al. (2012). Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30, 980-988.
- Kwok, M., Rawstron, A.C., Varghese, A., Evans, P.A., O'Connor, S.J., Doughty, C., Newton, D.J., Moreton, P., and Hillmen, P. (2016). Minimal residual disease is an independent predictor for 10-year survival in CLL. Blood 128, 2770-2773.
- Moreton, P., Kennedy, B., Lucas, G., Leach, M., Rassam, S.M., Haynes, A., Tighe, J., Oscier, D., Fegan, C., Rawstron, A., et al. (2005). Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 23, 2971-2979.
- Rawstron, A.C., Fazi, C., Agathangelidis, A., Villamor, N., Letestu, R., Nomdedeu, J., Palacio, C., Stehlikova, O., Kreuzer, K.A., Liptrot, S., et al. (2016). A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 30, 929-936.
- Rawstron, A.C., Villamor, N., Ritgen, M., Bottcher, S., Ghia, P., Zehnder, J.L., Lozanski, G., Colomer, D., Moreno, C., Geuna, M., et al. (2007). International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 21, 956-964.
- Wood, B., Jevremovic, D., Bene, M.C., Yan, M., Jacobs, P., Litwin, V., and Group, I.I.W. (2013). Validation of cell-based fluorescence assays: practice guidelines from the ICSH and ICCS - part V - assay performance criteria. Cytometry B Clin Cytom 84, 315-323.