Welcome to Navigate BioPharma
At Navigate BioPharma, we are more than just a testing laboratory. We design innovative biomarker solutions and customized validations based on intended use for clinical development from exploratory to regulated. Our approach is technology agnostic, meaning that we have a selection of platforms and techniques to ensure that you get the right answers. Our teams can apply our genomic, flow, digital pathology, or ligand binding analysis methods for across the spectrum of drug development and manufacturing applications.
Each result has an impact. Whether it is informing the treatment decision for a single patient, establishing pharmacodynamic understanding, or determining suitability of a manufacturing product to pass QC, we are passionate about delivering the right answer.
We are your partner throughout the approval process
We collaborate with our clients to achieve shared goals. With 15 years of clinical trial testing experience and extensive immunology expertise, we offer targeted solutions, end-to-end support, and quality oversight to mitigate the risks and challenges of the regulatory process.
Quality is embedded in all of our activities and is owned by each individual, to ensure we do what is right, not just when others are watching. This is the cornerstone of our positive interactions with regulators including EMA, HGRAC, FDA, and CDC.
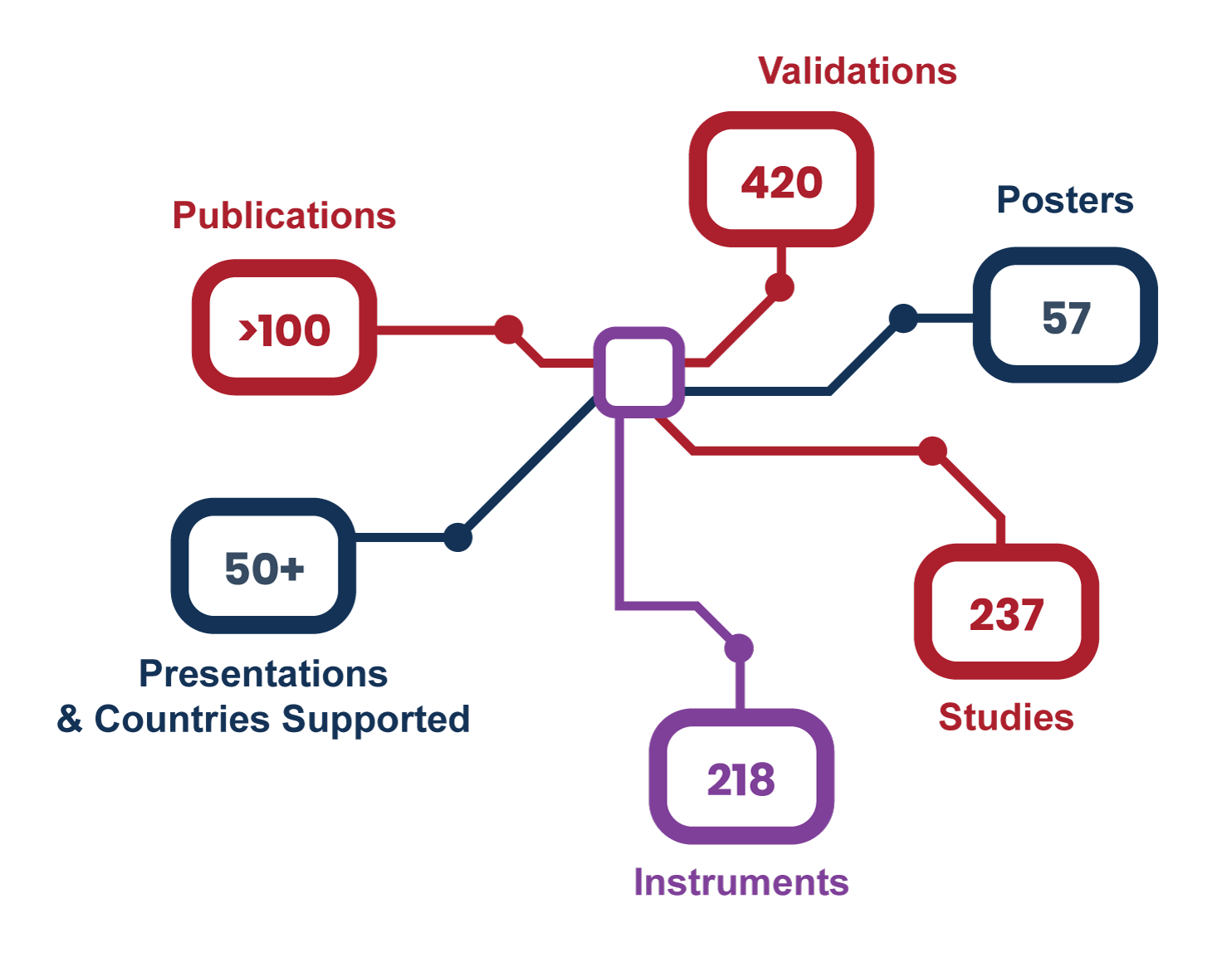
Our team has extensive therapeutic, disease, and technological expertise
Our deep disease knowledge and team of highly-trained specialists can help you determine which platform will provide the best assessment for your intended use. At Navigate, we know that there are complexities and nuances to the diseases you are developing therapies for, and that aligning the questions you need answered with the right analytical method(s) for a particular patient population is critical.