Cell and gene therapy
We have the longest tenure in the industry with multiple years of assisting successful Cell & Gene Therapy (CGT) product launches. Our experience and regulatory knowledge will deliver critical data helping you successfully move your product from preclinical development through your manufacturing needs. In addition, we provide a complete analytical workflow that provides key insights to complex clinical questions. Our analytical tools and best practices ensure the most efficient clinical development timelines without sacrificing quality standards.
Our extensive experience in immune directed therapies includes CAR-T or TCR, gene therapies, iRNA therapies, bi/tri-specific antibodies and selective kinase inhibitors.
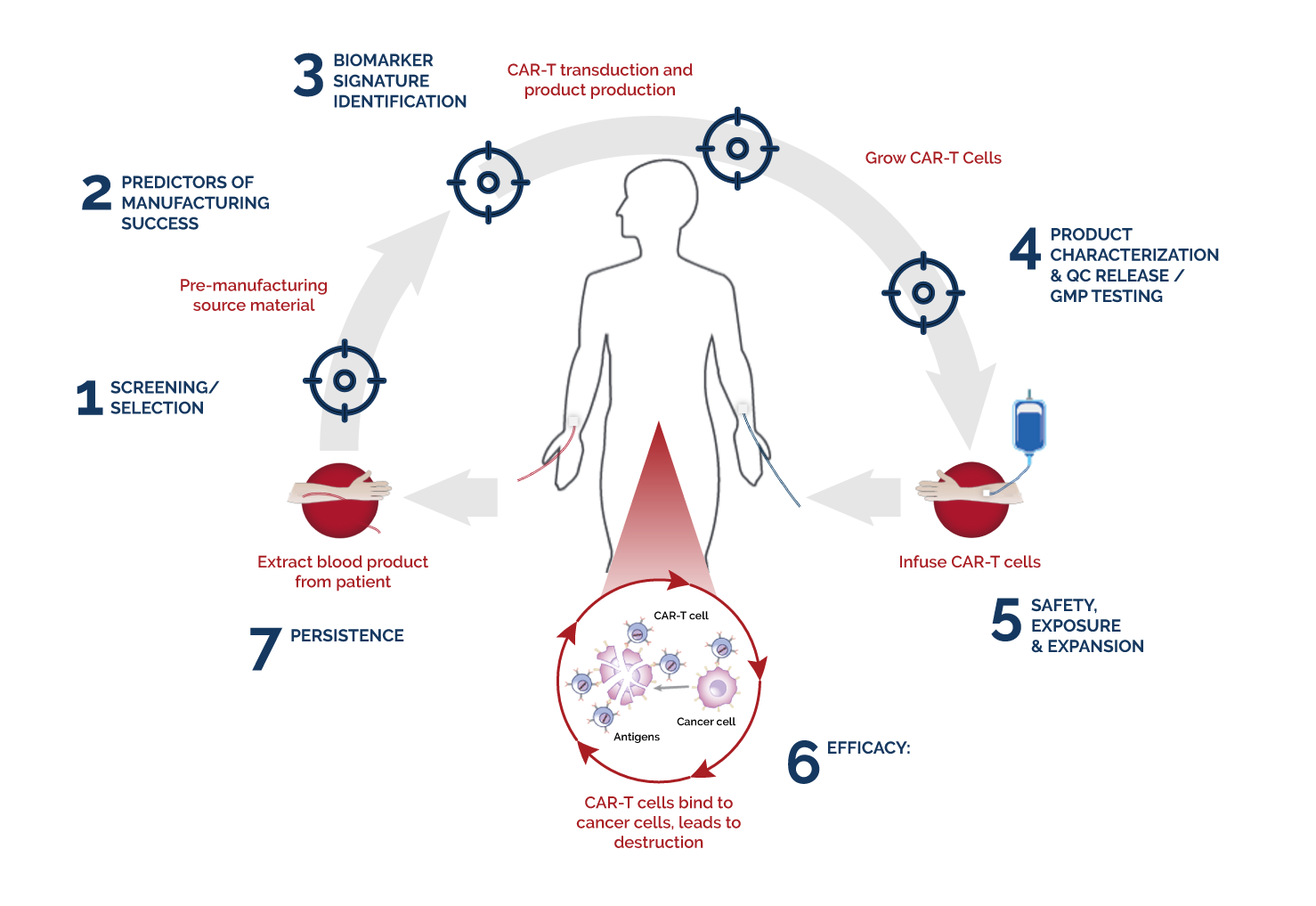
Best practices for the development, analytical validation and clinical implementation of flow cytometric methods for chimeric antigen receptor T cell analyses
doi.org/10.1002/cyto.b.21985
Monitoring CAR-T cell kinetics in clinical trials by multiparametric flow cytometry: Benefits and challenges
https://doi.org/10.1002/cyto.b.21891
Oncology/hematology
Oncology
We look at cancer holistically, from the molecular levels to the whole body. Our team’s expertise in cancer biology helps you determine the optimal pathway to treatment development and regulatory approval. Additionally, our advanced technological platforms make it possible for you to execute on a variety of complex clinical trials.
Hematological disorders
By leveraging our world class expertise in flow cytometry, molecular testing, and ligand binding technologies, we provide the complete clinical package- from drug-target interactions to genetic characterization of patients for enrollment and critical minimal residual disease read-outs.
Rare Diseases
Rare disease
Rare disease is an area where detailed data is urgently important for disease diagnosis and treatment research. Our expertise can provide the support and solutions you need to overcome the complex and unique challenges of these ultra-rare conditions.
